
Aldevron Now Offering AAV Rep/Cap Plasmids for Viral Vector Production
Expanding Catalog of Inventory Products Helps Reduce Timeline and Cost
FARGO, N.D.--(BUSINESS WIRE)--#Aldevron--Aldevron announced today the immediate availability of rep/cap plasmids pALD-AAV2, AAV5 and AAV6 to support AAV viral vector manufacturing. All three rep/cap plasmids are immediately available at Research Grade. Additionally, pALD-AAV6 is immediately available at the GMP-Source® quality level, while pALD-AAV2 and pALD-AAV5 will be made available based on market demand. The plasmids are royalty free for all applications in support of pre-clinical activities through clinical development and commercial production.
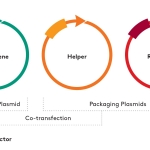

The addition of these three rep/cap plasmids expands Aldevron’s suite of ready-to-use and royalty-free constructs in support of AAV manufacture. They join Aldevron’s helper plasmid, pALD-X80, which is also available for pre-clinical through commercial applications.
“Genetic medicine has experienced tremendous growth, particularly over the past several years. Aldevron is proud to continue our support for the field of advanced therapies by developing more products that are readily available, helping bring life-saving treatments to market,” said Michelle Berg, President of Aldevron’s GMP Nucleic Acids Business Unit. “Rep/cap plasmids are one of three key components for AAV manufacturing and with Aldevron's ready-to-ship offerings, clients do not need custom manufacture, thereby reducing timeline and cost. With no royalty fees through commercial production, these products provide our clients a clearer path to better enable these potential approaches to reach patients.”
Berg added that Aldevron’s 22 years of experience with custom manufacturing high-quality products used by biotech companies around the globe ensures the off-the-shelf products it produces are stable and reliable. “Along with offering catalog items, we have increased capacity and reduced lead times, providing us the ability to scale while meeting important client deadlines.”
In addition to the AAV suite of constructs, Aldevron has a catalog of products including lentivirus plasmids, IVT enzymes, and a variety of CRISPR nucleases.
About Aldevron
Aldevron is a privately held company founded in 1998. It serves the biotechnology industry with custom production of nucleic acids and proteins. Thousands of clients use Aldevron-produced plasmids, RNA and gene editing enzymes for projects ranging from discovery research to clinical trials to commercial applications. These products are critical raw materials and key components for vaccine development, gene and cell therapy, immunotherapy, agricultural biotech, veterinary medicines, molecular diagnostics and more. Aldevron is known for inventing the GMP-Source® quality system, and for specializing in GMP manufacturing, operating the world’s largest facility at its Company headquarters in Fargo, North Dakota. The company’s protein development and manufacturing operations are located in Madison, Wisconsin. To learn more, visit www.aldevron.com.
Contacts
Ellen Shafer
Senior Director of Marketing and Communications
ellen.shafer@aldevron.com
701.297.9256
Editor Details
-
Company:
- Businesswire
