
Global eCOA, eSource & Clinical Trials Market: Emerging Economies Expected to Influence Growth until 2029
The latest Fact.MR study indicates healthcare facilities, contract research organizations, and educational & research institutes collectively spent ~US$ 1,950 Mn on eCOA, eSource and clinical trials solutions in 2018. The eCOA, eSource & clinical trials market is envisaged to witness substantial traction through 2019, with gains primarily driven by the growing penetration of digitization in healthcare sector and substantial increase in pharma spending on clinical trials.
Rapidly expanding deployment of clinical trial solutions, which contributed to ~40% of the total spending on eCOA, eSource, and clinical trials in 2018, will continue to appeal a widening bandwidth of institutions through 2029, says the report.
For detailed insights on enhancing your product footprint, request for a sample here https://www.factmr.com/connectus/sample?flag=S&rep_id=4042
Spending on eCOA Set to Quadruple
The Fact.MR study opines that though the deployment of clinical trial solutions overshadowed the use of Electronic Clinical Outcome Assessment (eCOA), eSource, and EDA in 2018, the market is envisaged to witness a range of changes during the foreseeable period. Growing penetration of Bring Your Own Device (BYOD) trend in clinical trials is highly likely to pace up the use of eCOAs, such as ePROS, ClinROs, ObsROs, and PerfORs across wide range of pharmaceutical companies and research facilities.
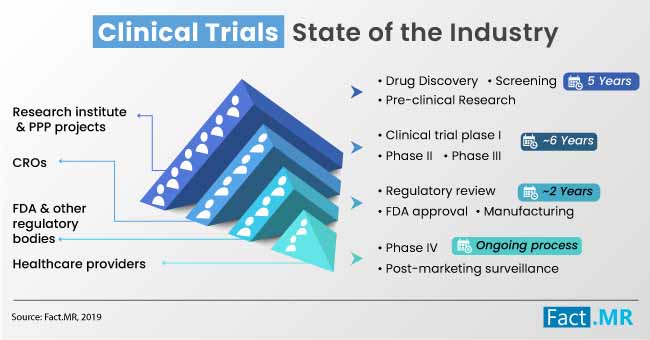
eCOAs is envisaged to hold ~45% of the overall spending share by the end of 2029, in line with the growing use of smartphones and tablets to seamlessly collect clinical data for analysis. Additionally, reduced cost and high scalability of eCOA are the primary determinants that continue to push its adoption during clinical trials, which is envisaged to contribute significantly to the growth of the eCOA, eSource & clinical trials market
For critical insights on this market, request for methodology here https://www.factmr.com/connectus/sample?flag=RM&rep_id=4042
Developed Regions Spearhead in Clinical Trials Deployment Rate
The rapidly advancing healthcare infrastructure, fast internet connectivity, and stringent regulatory product approval framework continue to make North America and Europe a hotbed of opportunities for stakeholders. With an increased number of companies getting cloud based (SaaS) deployment of clinical trial solutions in developed regions, owing to smooth internet connectivity, the market continues to accelerate at a steady pace. Cloud based deployment of digitized solutions accounted for 64% of the overall spending in Europe.
As per the study North America eCOA, eSource & clinical trials spending has been witnessing a steady growth over the years, with the US spending expected to account for ~90% revenue share in the regional eCOA, eSource & clinical trials spending in 2018. In view of the manifold cost and processing benefits of digitization in clinical trials, a larger number of pharmaceutical companies, contract research organizations, and education & research institutions have shifted their focus on paperless approaches. Growing adoption of clinical trial solutions, which contributed ~40% revenue share in North America eCOA, eSource, and clinical trials market, continue to create a window of opportunities of growth for market players.
For in-depth competitive analysis, buy now – https://www.factmr.com/checkout/4042
Fact.MR study provides the long-term outlook of the eCOA, eSource, & clinical trials market for 2019 to 2029. The eCOA, eSource, & clinical trials market is envisaged to register a CAGR of ~14% through 2029.
Chapter 01 – eCOA, eSource & Clinical Trials Market – Executive Summary
The report commences with an executive summary of the eCOA, eSource & Clinical Trials market report, which includes a summary of the key findings and key statistics of the market. It also includes the market spending (US$ million) estimates of the leading segments of the eCOA, eSource & Clinical Trials market and market trends.
Chapter 02 – eCOA, eSource & Clinical Trials Market – Market Introduction
Market introduction comprises detailed market definitions. Readers can find detailed taxonomy and the definitions associated with the eCOA, eSource & Clinical Trials market in this chapter, which will help them gather the basic information about the eCOA, eSource & Clinical Trials market.
Read More Trending and Similar Reports from Fact.MR – https://www.globenewswire.com/en/news-release/2019/05/14/1823788/0/en/Smartphone-based-Pregnancy-Point-of-Care-Testing-Emerging-as-a-Potential-Alternative-to-Lab-based-Diagnostics-Fact-MR.html
Explore Fact.MR’s Comprehensive Coverage on Healthcare Landscape
Wound Tissue Analysis Solutions Market Forecast, Trend Analysis & Competition Tracking – Global Market Insights 2018 to 2028- https://www.factmr.com/report/2189/wound-tissue-analysis-solutions-market
Wireless Neurostimulator Market Forecast, Trend Analysis & Competition Tracking – Global Market Insights 2018 to 2028- https://www.factmr.com/report/2188/wireless-neurostimulator-market
Skin Cooling Systems Market Forecast, Trend Analysis & Competition Tracking – Global Market Insights 2018 to 2028- https://www.factmr.com/report/2186/skin-cooling-systems-market
About Us:
Market research and consulting agency with a difference! That’s why 80% of Fortune 1,000 companies trust us for making their most critical decisions. While our experienced consultants employ the latest technologies to extract hard-to-find insights, we believe our USP is the trust clients have on our expertise. Spanning a wide range – from automotive & industry 4.0 to healthcare & retail, our coverage is expansive, but we ensure even the most niche categories are analyzed. Our sales offices in United States and Dublin, Ireland. Headquarter based in Dubai, UAE. Reach out to us with your goals, and we’ll be an able research partner.
Contact:
US Sales Office:
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583
Corporate Headquarter:
Unit No: AU-01-H Gold Tower (AU),
Plot No: JLT-PH1-I3A,
Jumeirah Lakes Towers,
Dubai, United Arab Emirates
Email: sales@factmr.com
Visit Our Website: https://www.factmr.com
The post Global eCOA, eSource & Clinical Trials Market: Emerging Economies Expected to Influence Growth until 2029 appeared first on Latest Market Reports.
Editor Details
-
Company:
- MARKITWIRED Latest Market Reports
- Website:
