
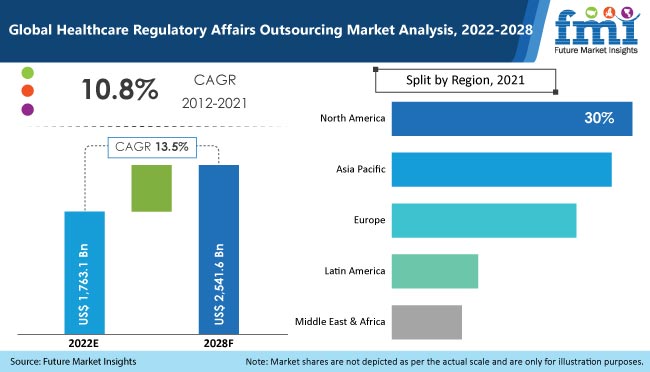
Healthcare Regulatory Affairs Outsourcing Market is slated to total US$ 1.76 Billion in 2022
[140 Pages Report] Consistent evaluation of cost-saving options such as regulatory affairs outsourcing is carried out by several drug/device manufacturers in order to streamline their operations and ensure product safety and maintain public healthcare.
The global health care regulatory affairs outsourcing market includes services like regulatory writing and publishing, clinical trial applications, etc. A new research report by Future Market Insights provides an in-depth analysis of the global regulatory affairs outsourcing market. This comprehensive research report is titled ‘Healthcare Regulatory Affairs Outsourcing Market: Global Industry Analysis, Size, Share, Growth, Trends and Forecast 2022 – 2028’.
It includes all the crucial numbers of the market along with the dynamics impacting it. According to this research report the global healthcare regulatory affairs outsourcing market is expected to reach a market revenue of over US$ 2.54 Bn by the end of 2028, growing at a stellar CAGR of 13.5% over the forecast period. The growth of the market is triggered largely by the increasing investments in the healthcare industry along with the increase in research and developments taking place.
Request a sample of this report@ https://www.futuremarketinsights.com/reports/sample/rep-gb-6355
Also increasing focus on CMOs and CROs has been observed in the market. The reports also depicts that Asia Pacific is expected to lead the global healthcare regulatory affairs outsourcing market during 2022-2028. Low cost, fast turnaround time and easy availability of the skilled and trained professionals will further result in increased outsourcing in APAC countries.
Global Healthcare Regulatory Affairs Outsourcing Market: Segmental Analysis
Based on End Users, mid-size pharmaceutical companies leads the market with a high market size expected during the forecast period, growing at a CAGR of 13.8% during the forecast period. However Biotechnology companies are also progressing at a high rate and is expected to give a strong competition to mid-size pharma companies in terms of growth rate expected during the forecast period.
On the basis of Services, regulatory writing and publishing service segment is expected to dominate the market with a market share of over US$ 800 Mn by the end of 2028. However the segment is much behind the other services in terms of growth rate. Regulatory consulting and legal representation is the service which is expected to register fastest growth at a CAGR of 14.3% during the forecast period.
Ask An Analyst @ https://www.futuremarketinsights.com/ask-the-analyst/rep-gb-6355
In terms of Region, Asia Pacific is expected to be the largest region with a high market valuation. However, North America is also expected to give tough competition to APAC. Both these regions are expected to reflect a valuation higher than US$ 720 Mn by 2028 end. However North America lags behind in the race with comparatively less CAGR expected during the forecast period. Whereas, Asia Pacific is expected to register the fastest growth rate of 13.2% CAGR during the forecast period.
Preview Analysis on Healthcare Regulatory Affairs Outsourcing Market Segmentation By Services Type – Regulatory Writing and Publishing, Regulatory Submissions, Clinical Trial Applications, and Product Registrations, Regulatory Consulting and Legal Representation, Other Regulatory Affairs; By End Users Type – Mid-Size Pharmaceutical, Companies,Large Pharmaceutical Companies, Biotechnology Companies, Medical Devices Manufacturer, Food & Beverage
Global Healthcare Regulatory Affairs Outsourcing Market: Competitive Landscape
The research report consists of a brief profile of all the major players leading in the industry. It also includes a SWOT analysis of these companies. The leading players mentioned in the report includes companies like Clinilabs, Inc., Accell Clinical Research, LLC., Freyr Solutions, The Weinberg Group Inc., Covance, Inc., (LabCorp), Pharmaceutical Product Development LLC, ICON plc., Sciformix Corporation etc.
For Information On The Research Approach Used In The Report, Request TOC@ https://www.futuremarketinsights.com/toc/rep-gb-6355
Healthcare Regulatory Affairs Outsourcing Market by Category
Services:
- Regulatory Writing and Publishing
- Regulatory Submissions
- Clinical Trial Applications
- Product Registrations
- Regulatory Consulting and Legal Representation
End User:
- Mid-Size Pharmaceutical Companies
- Large Pharmaceutical Companies
- Biotechnology Companies
- Medical Devices Manufacturer
- Food & Beverage Companies
Region
- North America
- Latin America
- Europe
- East Asia
- South Asia
- Oceania
- Middle East & Africa
About FMI:
Future Market Insights (ESOMAR certified market research organization and a member of Greater New York Chamber of Commerce) provides in-depth insights into governing factors elevating the demand in the market. It discloses opportunities that will favor the market growth in various segments on the basis of Source, Application, Sales Channel and End Use over the next 6-years.
Contact Us:
Future Market Insights,
Unit No: 1602-006, Jumeirah Bay 2, Plot No: JLT-PH2-X2A,
Jumeirah Lakes Towers, Dubai,
United Arab Emirates
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs
Editor Details
-
Company:
- MARKITWIRED
- Website:
