
Multiply Labs Collaborates with Industry Leaders University of California San Francisco, Cytiva, Thermo Fisher Scientific and Charles River to Deliver a Successful Proof-of-Concept of Robotic Cell Therapy Manufacturing
Multiply Labs collaborated with these life science industry leaders to demonstrate automated cell therapy manufacturing tasks, with an emphasis on cell expansion. The results of the proof-of-concept support the reproducibility of manual processes by robotic systems.
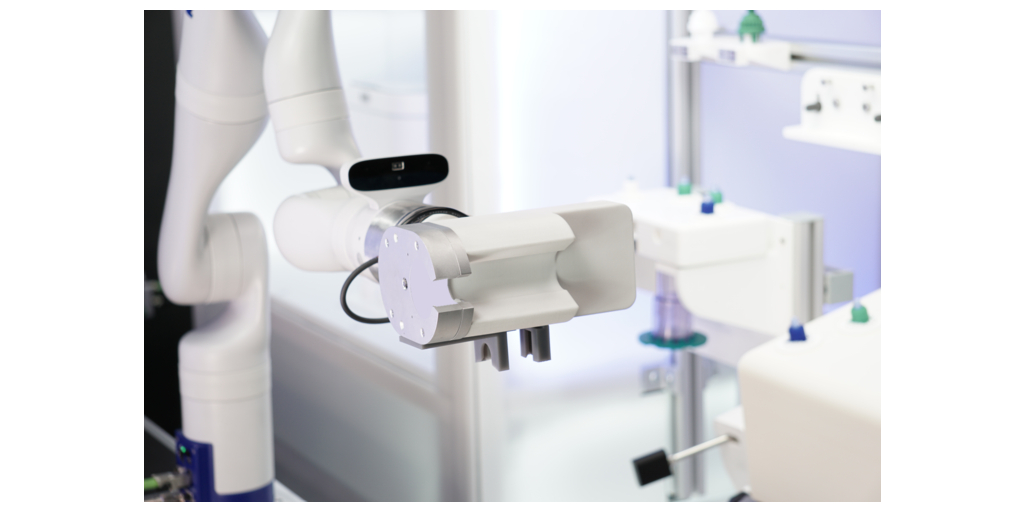


SAN FRANCISCO--(BUSINESS WIRE)--#manufacturing--Multiply Labs, a robotics company developing industry-leading automated manufacturing systems to produce individualized drugs (including but not limited to cell therapies), announced today that its proof-of-concept robotic system, developed along with UCSF, Cytiva, Thermo Fisher Scientific and Charles River, has been successfully completed. The data from this project indicate that manual cell therapy manufacturing tasks (and in particular, cell expansion processes based on leading GMP bioreactors) can be replicated by robotic systems by leveraging automation to achieve comparable critical process parameters.
The goal of the collaboration has been to develop an automation approach focused on 1) compatibility with market-leading, GMP-proven instruments, 2) modularity, which gives it the flexibility to implement a wide range of processes, and 3) efficiency, achieved through the parallelization of process bottlenecks.
Cell therapy process development and manufacturing have traditionally been challenging and labor-intensive. Cell therapy manufacturing processes are dominated by extremely manual and repetitive tasks, where the risk of human error is high. Additionally, the highly skilled labor required to develop and manufacture cell therapies is expensive coupled with the difficulty around hiring, training, and retaining talent. The cell therapy process is complex, creating a challenging environment to achieve sustained levels of equipment and production floor utilization, placing increasing strain on manufacturing facilities. Simultaneously, the impressive clinical outcomes of gene modified cell therapies are driving significant demand, and further compounding the industry’s manufacturing bottlenecks.
Multiply Labs and its collaborators deployed a proof-of-concept robotic system at UCSF to demonstrate this technology through the automation of a representative cell therapy expansion process. The deployment of the robotic systems at UCSF was carried out within a sponsored research agreement. The key goal of this project was to show that a robotic system can successfully replicate a set of critical manual tasks in a cell therapy manufacturing process by automating the operation of the same GMP-proven instruments, consumables and reagents. This approach shows that it is possible to automate an existing cell expansion process without modifying it.
“If you had to change a process in order to automate it, the benefits of automation (efficiency and quality) would need to be weighed against the regulatory and scientific drawbacks connected with modifying a validated process,” said Fred Parietti, PhD, Co-Founder and CEO of Multiply Labs. “With Multiply Labs’ technology, adopting automation does not require this trade-off.”
The data collected by the proof-of-concept indicates that a complex, manual cell expansion process can be automated using Multiply Labs’ technology. The critical process parameters of the robotic process track the ones of the manual process, and the results of the manual and robotic processes are not statistically different. Multiply Labs and its collaborators plan to discuss this data at the 2023 Cell and Gene Meeting on the Mesa and in a forthcoming scientific publication. “Charles River is always looking for new technologies to increase the speed and efficiency of process development and manufacturing cell therapies,” said Matt Hewitt, Vice President, Technical Officer CGT & Biologics at Charles River. “The combination of Multiply Labs’ unique robotic technology with Charles River’s industry-leading process expertise has the potential to do just that.”
The proof-of-concept data were focused on an initial set of industry-leading, GMP-ready instruments, including the Xuri™ bioreactor (manufactured by Cytiva) and the Thermo Scientific™ Heracell™ VIOS incubator (manufactured by Thermo Fisher Scientific).
“We are pleased by this collaboration with Multiply Labs, which demonstrated that our industry-leading bioreactors are key to successfully automate validated cell therapy processes,” said Caroline Rand, Strategy and Business Development Leader – Cell Therapy at Cytiva.
“The Consortium is leading the way in the automation of cell therapy manufacturing and we’re proud that our industry standard incubators and reagents have integrated seamlessly with this initiative. Through collaborations like this, we can optimize workflows and increase scalability which will help expedite the delivery of these potentially curative therapies to more patients,” added Xavier De Mollerat Du Jeu, Senior Director of Research and Development at Thermo Fisher Scientific.
About Multiply Labs
Multiply Labs is a robotics company that provides autonomous manufacturing technology to the pharmaceutical industry. The company develops advanced, cloud-controlled robotic systems that enable the production of individualized drugs at scale. Its customers include some of the largest global organizations in the advanced pharmaceutical manufacturing space. Multiply Labs’ expertise is at the intersection of robotics and biopharma – its team includes mechanical engineers, electrical engineers, computer scientists, software engineers and pharmaceutical scientists. The founding team got in touch because of their shared love of robots at MIT. The company is based in San Francisco, California. For more information, please visit www.multiplylabs.com.
About UCSF
The University of California, San Francisco (UCSF) is exclusively focused on the health sciences and is dedicated to promoting health worldwide through advanced biomedical research, graduate-level education in the life sciences and health professions, and excellence in patient care. UCSF Health, which serves as UCSF’s primary academic medical center, includes top-ranked specialty hospitals and other clinical programs, and has affiliations throughout the Bay Area. UCSF School of Medicine also has a regional campus in Fresno. Learn more at https://ucsf.edu or see our Fact Sheet.
About Cytiva
At Cytiva, our mission is to advance and accelerate the development of therapeutics. With nearly 16,000 associates in more than 40 countries, we’re driven to use our expertise and talent to achieve better flexibility, capacity, and efficiency for our customers. Our broad and deep portfolio of tools and technologies, global scale, and best-in-class service provides critical support from discovery to delivery, for customers spanning researchers, emerging biotech, large-scale biopharma, and contract manufacturers. Learn more at cytiva.com.
About Thermo Fisher Scientific
Thermo Fisher Scientific Inc. is the world leader in serving science, with annual revenue of over $40 billion. Our Mission is to enable our customers to make the world healthier, cleaner and safer. Whether our customers are accelerating life sciences research, solving complex analytical challenges, increasing productivity in their laboratories, improving patient health through diagnostics or the development and manufacture of life-changing therapies, we are here to support them. Our global team delivers an unrivaled combination of innovative technologies, purchasing convenience and pharmaceutical services through our industry-leading brands, including Thermo Scientific, Applied Biosystems, Invitrogen, Fisher Scientific, Unity Lab Services, Patheon and PPD. For more information, please visit www.thermofisher.com.
About Charles River
Charles River provides essential products and services to help pharmaceutical and biotechnology companies, government agencies and leading academic institutions around the globe accelerate their research and drug development efforts. The company’s dedicated employees are focused on providing clients with exactly what they need to improve and expedite the discovery, early-stage development, and safe manufacture of new therapies for the patients who need them. To learn more about Charles River’s comprehensive portfolio and breadth of services, visit www.criver.com.
Contacts
Marae Hartwell
info@multiplylabs.com
Editor Details
-
Company:
- Businesswire
