
Global Transcatheter Mitral Valve Market is expected to grow US$ 3.3 Billion by 2032, developed in collaboration with Abbott, a healthcare company | FMI
 Transcatheter Mitral Valve Market
Transcatheter Mitral Valve Market
The global transcatheter mitral valve market size is experiencing an unprecedented surge, setting the stage for remarkable growth and innovation. According to comprehensive market research, the industry is projected to reach a valuation of US$ 933.9 Million by the end of 2022, with an anticipated compound annual growth rate (CAGR) of 13.5%. This upward trajectory is expected to propel the market to approximately US$ 3.3 Billion by 2032.
This exponential growth can be attributed to groundbreaking advancements in medical technology, coupled with the increasing prevalence of cardiovascular diseases on a global scale. As a result, transcatheter mitral valve procedures are witnessing a substantial rise in demand, driven by their minimally invasive nature, providing patients with a viable alternative to traditional open-heart surgeries.
Request A Report Sample To Gain Comprehensive Insights! https://www.futuremarketinsights.com/reports/sample/rep-gb-15165
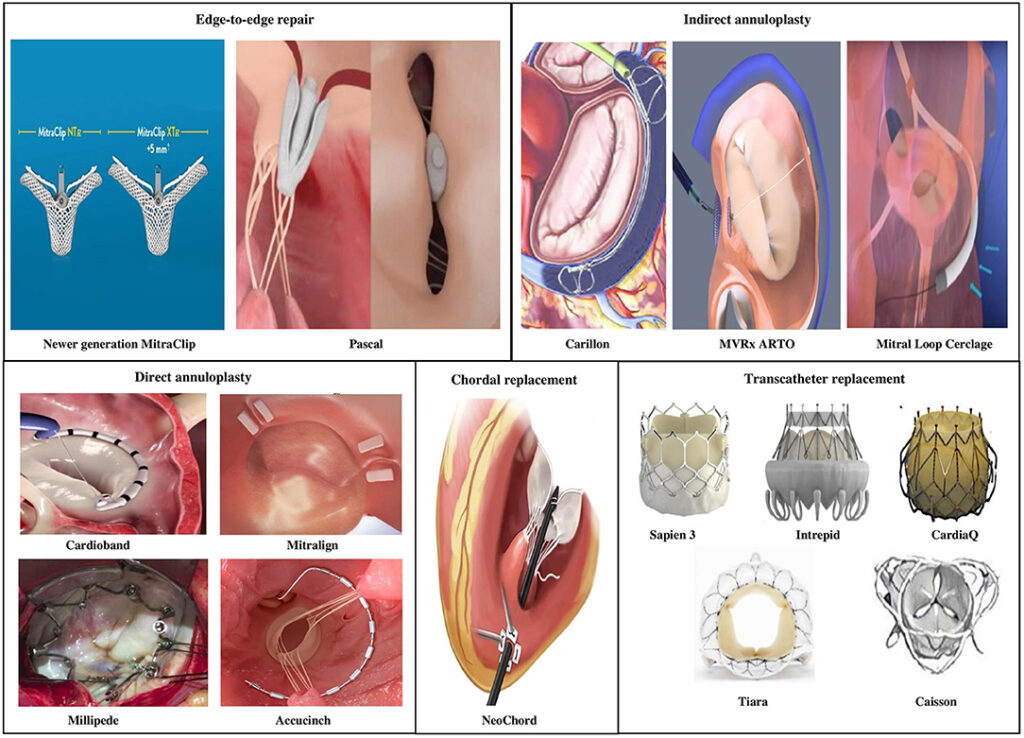
Edwards Lifesciences is a prominent medical device company that specializes in cardiovascular technologies. One of its notable products is the Edwards Lifesciences Transcatheter Mitral Valve (TMV) system, developed in collaboration with Abbott, a global healthcare company.
Product Overview:
The Edwards Lifesciences Transcatheter Mitral Valve (TMV) system is a breakthrough innovation designed to address mitral valve disorders, offering a less invasive treatment option compared to traditional open-heart surgery. The mitral valve is a crucial component of the heart’s function, ensuring proper blood flow between the heart’s left atrium and left ventricle. When this valve becomes dysfunctional due to conditions such as mitral regurgitation or mitral stenosis, it can lead to serious health complications.
Key Features and Benefits:
- Minimally Invasive Approach: The TMV system enables physicians to repair or replace the mitral valve without the need for open-heart surgery. This approach significantly reduces the risks associated with traditional surgical interventions, such as infection, blood loss, and extended recovery times.
- Transcatheter Delivery: The TMV system is delivered via a catheter-based procedure, typically performed through small incisions in the groin or chest. This approach eliminates the need for sternotomy (chest incision) and cardiopulmonary bypass, leading to shorter hospital stays and quicker patient recovery.
- Customized Treatment: The system offers a range of valve sizes and configurations to accommodate variations in patient anatomy and pathology, allowing for personalized treatment plans tailored to each individual’s needs.
- Durable and Biocompatible Materials: The TMV system incorporates advanced materials that are durable, biocompatible, and designed to withstand the dynamic conditions within the heart, ensuring long-term functionality and reducing the likelihood of complications such as thrombosis or tissue overgrowth.
- Proven Efficacy: Clinical studies have demonstrated the safety and efficacy of the TMV system in treating mitral valve disorders, with outcomes comparable to or even better than those of conventional surgical approaches in select patient populations.
Clinical Applications:
- Mitral Regurgitation (MR): The TMV system is particularly beneficial for patients with mitral regurgitation, a condition characterized by the backward flow of blood into the left atrium due to improper valve closure. By repairing or replacing the dysfunctional mitral valve, the TMV system helps restore normal blood flow and alleviate symptoms such as fatigue, shortness of breath, and heart palpitations.
- Mitral Stenosis: In cases of mitral stenosis, where the mitral valve becomes narrowed and obstructs blood flow from the left atrium to the left ventricle, the TMV system can provide relief by widening the valve opening and improving cardiac function.
Explore The Art of Informed Decision-Making Via Our Methodology: https://www.futuremarketinsights.com/request-report-methodology/rep-gb-15165
Competitive Landscape: Transcatheter Mitral Valve Market
The transcatheter mitral valve market is consolidated with the presence of various established players across the globe. Key players are enforcing clinical evaluations of pipelined products to strengthen their market positions. They are also investing huge sums in research and development activities to generate more revenues in the global market.
- In March 2022, Edwards Lifesciences received approval from the U.S. Food and Drug Administration (FDA) for its MITRIS RESILIA valve. It is a tissue valve replacement which is specifically designed for the heart’s mitral position.
- In April 2020, Abbott received the CE Mark approval for its Tendyne Transcatheter Mitral Valve Implantation System and TriClip Transcatheter Tricuspid Valve Repair System under its structural heart device portfolio.
Transcatheter Mitral Valve Market Outlook by Category:
By Product:
- Transcatheter Mitral Valve Replacement
- Transcatheter Mitral Valve Repair
By Indication:
- Mitral Stenosis
- Mitral Regurgitation
By End User:
- Hospitals
- Ambulatory Surgical Centers
- Cardiac Catheterization Laboratories
Act Now to Explore In-Depth Market Analysis: Purchase Now to Access Industry Trends: https://www.futuremarketinsights.com/checkout/15165
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 5000 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-845-579-5705
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Editor Details
-
Company:
- MARKITWIRED
- Website:
