
Clinical Trial Management System Market Projected To Grow At 10.3% CAGR, Crossing US$ 4.37 Billion By 2032, Reports Marketresearch.Biz
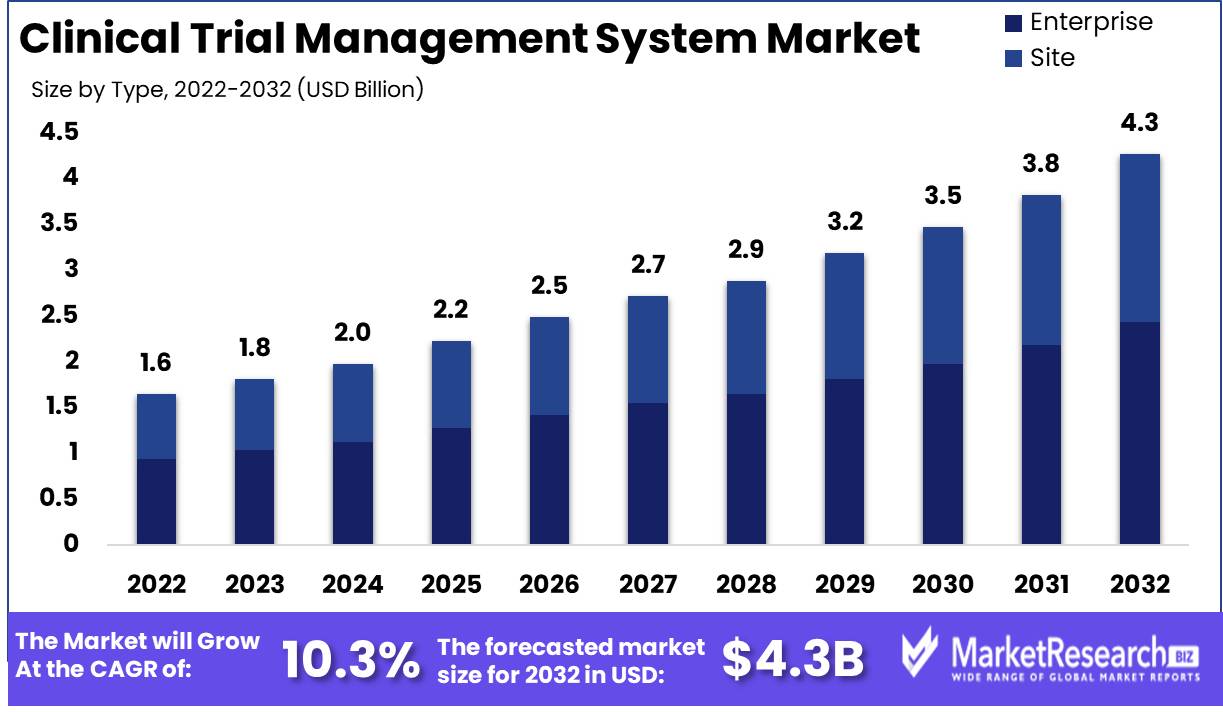
Marketresearch.biz reports that the Clinical Trial Management System Market size is expected to be worth around USD 4.37 Bn by 2032 from USD 1.64 Bn in 2022, growing at a CAGR of 10.3% during the forecast period from 2023 to 2032.
Overview of the Clinical Trial Management System (CTMS) Market
Clinical Trial Management Systems (CTMS) facilitate the planning, execution, and monitoring of clinical trials, streamlining processes and ensuring regulatory compliance. These systems integrate various functionalities, including participant recruitment, data management, and reporting, enhancing efficiency and accuracy in clinical research. The CTMS Market is driven by technological advancements, increasing clinical trial complexities, and regulatory requirements, shaping the landscape of clinical research.
Get Full PDF Sample Copy of Report (Including Full TOC, List of Tables & Figures, Chart) Click Here to Download a Sample Report: https://marketresearch.biz/report/clinical-trial-management-system-market/request-sample/
Driving Factors of the Clinical Trial Management System Market
- Efficiency and Cost Reduction: CTMS improves operational efficiency, reduces manual errors, and lowers overall trial costs.
- Regulatory Compliance: Compliance with stringent regulatory requirements, such as Good Clinical Practice (GCP), drives adoption of CTMS for data management and audit trails.
- Increasing Clinical Trial Complexity: Rising complexity of clinical trials, including large-scale studies and adaptive trial designs, necessitates robust CTMS for effective management.
- Globalization of Clinical Trials: Expansion of clinical trials to diverse geographic regions requires CTMS with multi-site capabilities and real-time data access.
- Technological Advancements: Innovations in CTMS software, such as cloud-based platforms and mobile applications, enhance accessibility and usability for trial stakeholders.
- Demand for Data-driven Insights: CTMS generates comprehensive data analytics and reporting, enabling informed decision-making and strategic planning in clinical research.
Restraining Factors of the Clinical Trial Management System Market
- Integration Challenges: Integration of CTMS with existing clinical trial systems and electronic health records (EHRs) may pose technical challenges and compatibility issues.
- Security and Privacy Concerns: Data security risks and regulatory compliance issues related to patient confidentiality and data protection impact CTMS adoption.
- Resource Constraints: Limited resources and budget constraints in smaller research organizations may hinder investment in CTMS implementation and customization.
You can check In-Detail TOC from here: https://marketresearch.biz/report/clinical-trial-management-system-market/
The Clinical Trial Management System Market report provides a comprehensive exploration of the sector, categorizing the market by type, application, and geographic distribution. This analysis includes data on market size, market share, growth trends, the current competitive landscape, and the key factors influencing growth and challenges. The research also highlights prevalent industry trends, market fluctuations, and the overall competitive environment.
This report offers a comprehensive view of the Global Clinical Trial Management System Market, equipping stakeholders with the necessary tools to identify areas for industry expansion. The report meticulously evaluates market segments, the competitive scenario, market breadth, growth patterns, and key drivers and constraints. It further segments the market by geographic distribution, shedding light on market leadership, growth trends, and industry shifts. Important market trends and transformations are also highlighted, providing a deeper understanding of the market’s complexities. This guide empowers stakeholders to leverage market opportunities and make informed decisions. Additionally, it provides clarity on the critical factors shaping the market’s trajectory and its competitive landscape.
Following Key Segments Are Covered in Our Report
By Type
- Enterprise
- Site
By Delivery Mode
- Web-based
- Cloud-based
- On-premise
By Component
- Software
- Service
By End-User
- Pharmaceutical and Biotechnology Firms
- CROs
- Medical Device Firms
Top Key Players in Clinical Trial Management System Market
- Oracle Corporation
- International Business Machines Corporation
- MedNet Solutions, Inc.
- Wipro Limited
- Veeva Systems
- Bio-Optronics Inc.
- Cognizant Technology Solutions Corporations
- Medidata Solutions Inc.
- IQVIA Inc.
- DSG, Inc.
- Forte Research Systems, Inc.
Get Full PDF Sample Copy of Report (Including Full TOC, List of Tables & Figures, Chart) Click Here to Download a Sample Report: https://marketresearch.biz/report/clinical-trial-management-system-market/request-sample/
Regional Analysis of Clinical Trial Management System Market
- North America: Dominates the market with extensive pharmaceutical and biotechnology industry. The United States leads, driven by high R&D spending and stringent regulatory requirements. Key players offer advanced CTMS solutions to streamline clinical trial processes and ensure compliance with FDA regulations.
- Europe: Experiences significant growth in the CTMS market due to increasing clinical research activities. Countries like Germany and the UK lead, with strong regulatory frameworks and emphasis on data integrity. Rising demand for efficient trial management solutions fuels market expansion and adoption of cloud-based CTMS platforms.
- Asia Pacific: Emerges as a key market for CTMS with growing clinical trial outsourcing and expanding biopharmaceutical sector. Countries like China and India witness substantial market growth, driven by cost-effective trial management solutions and large patient pools. Rising investments in healthcare infrastructure support market expansion and adoption of CTMS technologies.
- Middle East: Shows promising growth in the CTMS market with increasing clinical research investments and collaborations. Countries like the UAE and Saudi Arabia lead, investing in healthcare innovation and clinical trial infrastructure. Rising demand for CTMS solutions drives market expansion and adoption of digital trial management platforms.
- Africa: Witnesses gradual growth in the CTMS market with rising clinical trial activities and investments in research infrastructure. Countries like South Africa lead, with efforts to improve clinical trial capabilities and attract international research collaborations. Rising awareness of CTMS benefits fuels market expansion and adoption.
For More Information or Qurey, Visit @ https://marketresearch.biz/report/clinical-trial-management-system-market/
Growth Opportunities in the Clinical Trial Management System (CTMS) Market
- Increasing Outsourcing of Clinical Trials: The pharmaceutical and biotechnology industries are increasingly outsourcing clinical trial activities to contract research organizations (CROs). This trend creates opportunities for CTMS providers as CROs seek efficient and scalable solutions to manage clinical trial data and processes.
- Expanding Clinical Research Activities: The growing number of clinical research studies, particularly in therapeutic areas such as oncology, immunology, and rare diseases, is driving demand for CTMS solutions. As the complexity and volume of clinical trial data increase, there is a greater need for robust CTMS platforms to streamline trial management and ensure compliance with regulatory requirements.
- Adoption of Cloud-Based CTMS Solutions: The adoption of cloud-based CTMS solutions is increasing as pharmaceutical companies and CROs seek flexible and scalable software platforms. Cloud-based CTMS solutions offer advantages such as remote accessibility, real-time data visibility, and simplified IT infrastructure management, driving market growth.
- Focus on Patient-Centric Clinical Trials: There is a growing emphasis on patient-centricity in clinical trial design and execution. CTMS solutions that offer patient engagement features, such as mobile applications for remote data collection and electronic patient-reported outcomes (ePRO), are in demand as sponsors strive to enhance patient recruitment, retention, and overall trial experience.
- Integration of Artificial Intelligence and Analytics: The integration of artificial intelligence (AI) and advanced analytics capabilities into CTMS platforms is driving innovation in the market. AI-powered features such as predictive analytics, risk-based monitoring, and data-driven insights enable sponsors and CROs to optimize trial protocols, identify trends, and make informed decisions, creating opportunities for CTMS vendors.
Trending Factors in the Clinical Trial Management System (CTMS) Market
- Shift towards Decentralized Clinical Trials: The COVID-19 pandemic has accelerated the adoption of decentralized clinical trial (DCT) approaches, which leverage remote monitoring and digital technologies to conduct trials outside of traditional clinical settings. CTMS solutions that support DCT methodologies, such as remote patient monitoring and virtual visits, are trending as sponsors seek to adapt to changing trial dynamics.
- Focus on Data Security and Compliance: With the increasing volume of clinical trial data and heightened regulatory scrutiny, there is a growing focus on data security and compliance in the CTMS market. CTMS vendors are investing in robust security features, such as encryption, audit trails, and user authentication, to protect sensitive trial data and ensure compliance with data privacy regulations.
- Integration with Electronic Health Records (EHR) Systems: The integration of CTMS platforms with electronic health records (EHR) systems is becoming increasingly important for seamless data exchange and interoperability. Integrated CTMS-EHR solutions enable sponsors to leverage existing patient data from healthcare providers, streamline data capture processes, and improve data accuracy and completeness in clinical trials.
- Adoption of Real-World Evidence (RWE) Solutions: The use of real-world evidence (RWE) to supplement traditional clinical trial data is gaining traction in drug development and regulatory decision-making. CTMS solutions that support the collection and analysis of real-world data, such as electronic medical records and patient registries, are trending as sponsors seek to generate additional evidence of treatment effectiveness and safety.
- Focus on Remote Site Monitoring: The adoption of remote site monitoring technologies, such as centralized monitoring and risk-based monitoring (RBM), is reshaping clinical trial monitoring practices. CTMS solutions with built-in remote monitoring capabilities, such as data visualization dashboards and centralized monitoring portals, are in demand as sponsors seek to optimize monitoring processes, reduce site visits, and improve trial efficiency.
Our comprehensive Market research report endeavors to address a wide array of questions and concerns that stakeholders, investors, and industry participants might have. The following are the pivotal questions our report aims to answer:
Industry Overview:
- What are the prevailing global trends in the Clinical Trial Management System Market?
- How is the Clinical Trial Management System Market projected to evolve in the coming years? Will we see a surge or a decline in demand?
Product Analysis:
- What is the anticipated demand distribution across various product categories within Clinical Trial Management System?
- Which emerging products or services are expected to gain traction in the near future?
Financial Metrics:
- What are the projections for the global Clinical Trial Management System industry in terms of capacity, production, and production value?
- Can we anticipate the estimated costs, profits, Market share, supply and consumption dynamics?
- How do import and export figures factor into the larger Clinical Trial Management System Market landscape?
Strategic Developments:
- What strategic initiatives and movements are predicted to shape the industry in the medium to long run?
Pricing and Manufacturing:
- Which factors majorly influence the end-price of Clinical Trial Management System products or services?
- What are the primary raw materials and processes involved in manufacturing within the Clinical Trial Management System sector?
Market Opportunities:
- What is the potential growth opportunity for the Clinical Trial Management System Market in the forthcoming years?
- How might external factors, like the increasing use of Clinical Trial Management System in specific sectors, impact the Market’s overall growth trajectory?
Historical Analysis:
What was the estimated value of the Clinical Trial Management System Market in previous years, such as 2022?
Key Players Analysis:
- Who are the leading companies and innovators within the Clinical Trial Management System Market?
- Which companies are positioned at the forefront and why?
Innovative Trends:
- Are there any fresh industry trends that businesses can leverage for additional revenue generation?
Market Entry and Strategy:
- What are the recommended Market entry strategies for new entrants?
- How should businesses navigate economic challenges and uncertainties in the Clinical Trial Management System Market?
- What are the most effective Marketing channels to engage and penetrate the target audience?
Geographical Analysis:
- How are different regions performing in the Clinical Trial Management System Market?
- Which regions hold the most potential for future growth and why?
Consumer Behavior:
- What are the current purchasing habits of consumers within the Clinical Trial Management System Market?
- How might shifts in consumer behavior or preferences impact the industry?
Regulatory and Compliance Insights:
- What are the existing and upcoming regulatory challenges in the Clinical Trial Management System industry?
- How can businesses ensure consistent compliance?
Risk Analysis:
- What potential risks and uncertainties should stakeholders be aware of in the Clinical Trial Management System Market?
External Impact Analysis:
- How are external events, such as geopolitical tensions or global health crises (e.g., Russia-Ukraine War, COVID-19), influencing the Clinical Trial Management System industry’s dynamics?
- This report is meticulously curated to provide a holistic understanding of the Clinical Trial Management System Market, ensuring that readers are well-equipped to make informed decisions.
About Company
MarketResearch .Biz, a division of Prudour Pvt Ltd, excels in providing thorough Market research and analytical services. With a strong history of reliability, our company has established itself as a trusted consulting agency and a source for custom Market research insights. At MarketResearch .Biz, we recognize the diverse needs of our clients and are equipped to offer reports tailored to their specific requirements. Our dedication extends beyond standard practices, ensuring that we consistently deliver top-notch insights and a comprehensive view of the Market landscape to our clients.
Mr. Lawrence John
Marketresearch.Biz (Powered By Prudour Pvt. Ltd.)
420 Lexington Avenue, Suite 300
New York City, NY 10170,
United States
Tel: +1 (347) 796-4335
lawrence@marketresearch.biz
inquiry@marketresearch.biz
Editor Details
-
Company:
- Wired Release
- Website:
