
Rare Disease Genetic Testing Market to Grow at 13.1% CAGR, Reaching New Heights by 2032
The Rare Disease Genetic Testing Market is projected to grow significantly, with its size expected to reach USD 3,051.6 million by 2032 from USD 918.94 million in 2023, at a compound annual growth rate (CAGR) of 13.1% during the forecast period from 2022 to 2032. This growth is driven by several factors including the increasing prevalence of rare diseases, advancements in genetic testing technologies, and heightened government initiatives aimed at improving diagnosis and treatment.
Expanding patient registries and the development of new genetic testing technologies such as Next-Generation Sequencing (NGS) are pivotal in driving market growth. NGS, which accounted for over 35% of the market share in 2022, enables comprehensive genetic testing that is crucial for diagnosing a wide range of rare diseases. Moreover, government initiatives like the US FDA’s program for rare neurodegenerative diseases and the UK Rare Disease Framework emphasize faster diagnosis and improved treatment access, further propelling the market.
However, challenges such as the high cost of genetic tests and the limited availability of trained healthcare personnel pose significant barriers. The cost factor particularly affects accessibility in low- and middle-income countries, potentially limiting market growth in these regions.
Recent developments in the market include strategic collaborations and technological advancements. For example, in 2022, Bionano Genomics launched the Rare Undiagnosed Genetic Disease (RUGD) initiative to support research and improve patient care, and Predicine Inc. received FDA approval for an NGS assay for tumor mutation profiling. These developments highlight the ongoing efforts to enhance genetic testing capabilities and accessibility.
Overall, the market’s growth is bolstered by technological advancements, increasing awareness and diagnosis of rare diseases, and supportive government policies, despite the challenges of cost and resource availability.
Key Takeaways
- The global Rare Disease Genetic Testing Market is projected to reach USD 3,051.6 million by 2032 from USD 918.94 million in 2023.
- The market is expected to grow at a CAGR of 13.1% from 2022 to 2032.
- Increasing prevalence of rare diseases due to the rising population drives demand for genetic testing.
- Technological advancements in genetic testing make it more accurate and effective, promoting its use.
- Innovations in rare disease diagnosis products fuel market growth.
- High costs of genetic testing limit access, especially in low- and middle-income countries.
- Limited treatment options for rare diseases reduce the perceived benefit of genetic testing.
- Endocrine and metabolism diseases are anticipated to grow at a CAGR of 21.0%.
- Immunological diseases hold the second-highest revenue share in the market.
- Next-Generation Sequencing (NGS) leads the technology segment, contributing over 35.22% of revenue.
- Whole Exome Sequencing (WES) is gaining prominence for identifying genetic causes of rare diseases.
- Molecular genetic testing had the largest market share in 2022, over 41.10%, and is expected to maintain the lead.
- Chromosomal genetic tests and biochemical genetic tests are also significant segments.
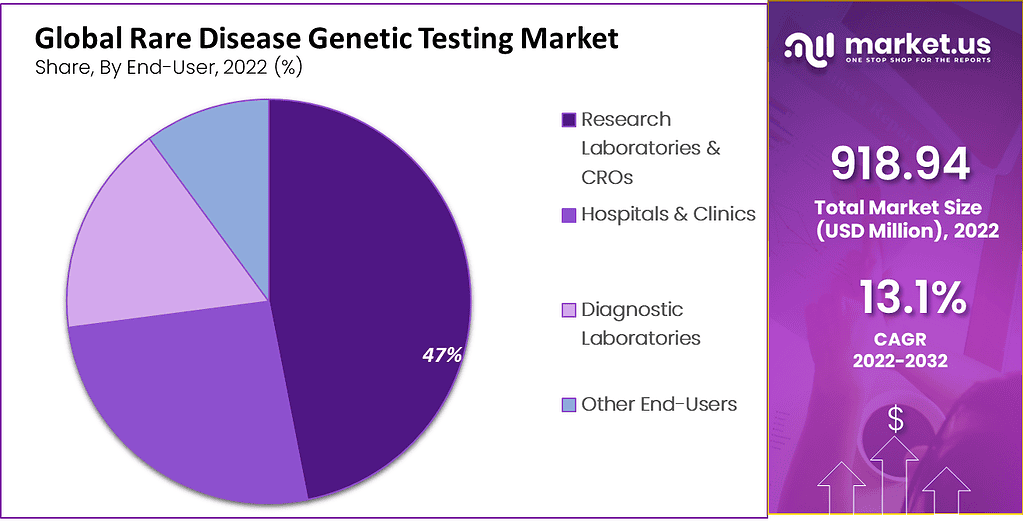
- Research labs and CROs held the largest market share in 2022, over 47%.
- Diagnostic labs are expected to grow at the fastest CAGR of 16.1% during the research period.
- Increasing demand for non-invasive genetic tests, such as blood-based and saliva-based tests, offers growth potential.
- Expansion into emerging markets, especially in Asia Pacific and MEA, can tap into the high prevalence of rare diseases.
- Next-Generation Sequencing (NGS) is increasingly used for genetic testing, offering comprehensive data on multiple genes.
- Whole-Exome Sequencing is growing in importance for clinical diagnosis and personalized medicine.
- North America led the market in 2022, with over 47.2% market share, driven by a high prevalence of rare diseases.
- Asia Pacific is expected to grow at the highest rate, with a CAGR of 18.1%, due to improving diagnostic awareness.
Get Sample PDF Report: https://market.us/report/rare-disease-genetic-testing-market/request-sample/
Rare Disease Genetic Testing Market Key Segments
Based on Disease Type
- Endocrine & Metabolism Diseases
- Immunological Disorders
- Neurological Disease
- Hematology Diseases
- Cancer
- Musculoskeletal Disorders
- Cardiovascular Disease
- Other Disease types
Based on Technology
- Next-Generation Sequencing( NGS)
- Whole Exome Sequencing
- Whole Genome Sequencing
- Array Technology
- PCR-Based Testing
- FISH
- Sanger Sequencing
- Karyotyping
- Other Technology
Based on Specialty
- Molecular Genetic Tests
- Chromosomal Genetic Tests
- Biochemical Genetic Tests
Based on End-User
- Research Laboratories & CROs
- Hospitals & Clinics
- Diagnostic Laboratories
- Other End-Users
Key Regions
- North America (The US, Canada, Mexico)
- Western Europe (Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, Rest of Western Europe)
- Eastern Europe (Russia, Poland, The Czech Republic, Greece, Rest of Eastern Europe)
- APAC (China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, Rest of APAC)
- Latin America (Brazil, Colombia, Chile, Argentina, Costa Rica, Rest of Latin America)
- Middle East & Africa (Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, Rest of MEA)
Buy Directly: https://market.us/purchase-report/?report_id=100151
Key Players Analysis
Quest Diagnostics is a leading provider of diagnostic information services, including comprehensive genetic testing for rare diseases. The company acquired Blueprint Genetics to enhance its capabilities in gene variant interpretation and next-generation sequencing. This acquisition enables Quest to offer highly specialized genetic insights, improving patient care and pharmaceutical drug development. Blueprint Genetics provides over 200 panel tests spanning 14 medical specialties, catering to the needs of patients with rare genetic conditions. Quest’s extensive infrastructure and advanced diagnostics ensure broad access to high-quality, actionable genetic data.
Centogene N.V. specializes in rare disease diagnostics and genetic testing, with a focus on transforming clinical and genetic data into actionable medical information. The company operates a global network of laboratories and provides comprehensive testing services, including whole exome sequencing, biomarker discovery, and patient-centric data repositories. Centogene’s proprietary CentoMD® platform integrates clinical and genetic data to support accurate diagnosis and treatment planning for rare diseases. By leveraging advanced bioinformatics, Centogene aims to accelerate the development of personalized therapies for patients worldwide.
Invitae Corp. is dedicated to bringing comprehensive genetic information into mainstream medical practice to improve healthcare for billions of people. The company offers a wide range of genetic tests, including those for rare diseases, and emphasizes accessibility and affordability. Invitae’s robust genetic testing services are supported by an integrated platform that combines advanced sequencing technology with extensive data analysis. This approach helps clinicians and patients make informed decisions about managing rare genetic conditions, enhancing diagnostic accuracy, and facilitating personalized treatment plans.
3billion Inc. focuses on diagnosing rare genetic disorders through whole exome sequencing and advanced bioinformatics. The company aims to make genetic testing more accessible and affordable, offering comprehensive diagnostic solutions that cover thousands of rare diseases. 3billion’s platform uses proprietary algorithms and extensive genetic databases to interpret sequencing data accurately. This enables precise diagnosis and supports the development of targeted therapies for rare genetic conditions, improving patient outcomes and advancing personalized medicine.
Arup Laboratories is a national reference laboratory offering extensive genetic testing services, including those for rare diseases. The company’s expertise in genetic diagnostics spans various medical specialties, with a strong focus on high-quality, reliable results. Arup utilizes state-of-the-art technologies, such as next-generation sequencing and chromosomal microarray analysis, to detect and interpret genetic variants associated with rare conditions. Their commitment to research and innovation ensures continuous improvement in diagnostic accuracy and patient care, supporting healthcare providers in managing rare genetic disorders effectively.
Rare Disease Genetic Testing Market Key Players:
- Quest Diagnostics Inc.
- Centogene N. V
- Invitae Corp
- 3billion Inc.
- Arup Laboratories
- Eurofins Scientific
- Strand Life Sciences
- Ambry Genetics
- Perkin Elmer Inc.
- Realm IDX Inc.
- Macrogen Inc.
- Baylor Genetics
- Color Genomics Inc.
- Health Network Laboratories
- PreventionGenetics
- Progenity Inc.
- Coopersurgical Inc.
- Fulgent Genetics Inc.
- Myriad Genetics Inc.
- Laboratory Corporation of America Holdings
- Opko Health Inc.
- Other key players
Rare Disease Genetic Testing Market Report Scope >> Market Value (2023): USD 918.9 Million || Forecast Revenue (2033): USD 3,051.6 Million || CAGR (2024-2033): 13.1% || Base Year Estimation: 2023 || Historic Period: 2019-2022 || Forecast Period: 2024-2033.
Inquire More about report: https://market.us/report/rare-disease-genetic-testing-market/#inquiry
About Market.US
Market.US is renowned for its comprehensive market research and analysis, providing customized and syndicated reports to a global clientele. Specializing in a variety of sectors, they offer strategic insights and detailed market forecasts, assisting businesses in making informed decisions. With a focus on innovation and accuracy, Market.US supports clients in over 126 countries, and maintains a strong repeat customer rate, underscoring their commitment to quality and client satisfaction. Their team excels in delivering exceptional research services, ensuring that no detail is overlooked in any target market.
Contact Details
Market.us (Powered By Prudour Pvt. Ltd.)
Contact No: +1 718 618 4351.
Email: inquiry@market.us
Blog: https://medicalmarketreport.com/
View More Trending Reports
Medical Adhesive Tapes Market Reach USD 16 Billion By 2032
RNA Analysis Market Expected To Expand USD 34.37 Billion By 2032
Internet of Medical Things (IoMT) Market Reach USD 370.9 Billion By 2032
Sexually Transmitted Disease Market Projected Surge To USD 171 Billion By 2032
Cell and Gene Therapy Market Projected To Reach USD 78.0 Billion With 22.6% CAGR By 2032
Editor Details
-
Company:
- Wired Release
- Website:
