
Regentis Biomaterials Commences Trading on NYSE: Set to Transform Cartilage Repair Market with Off-the-Shelf Regenerative Product
Lead product GelrinC®, a hydrogel synchronized erosion and resorbable implant, the only restorative product for knee cartilage repair, is a breakthrough effective and economical procedure to address a large unmet need
GelrinC is approved for knee cartilage repair in the European Union and is currently at midpoint in a pivotal FDA trial for the same indication to address a U.S. market of more than 470,000 potential cases annually
Upcoming expected catalysts include commercial launch in Europe and submission to FDA for approval in U.S.
HERZLIYA, IL / ACCESS Newswire / December 8, 2025 / Regentis Biomaterials Ltd., ("Regentis" or the "Company") (NYSE American:RGNT), a late clinical stage regenerative medicine company focused on innovative tissue repair solutions, is now trading on the NYSE American under the symbol "RGNT".
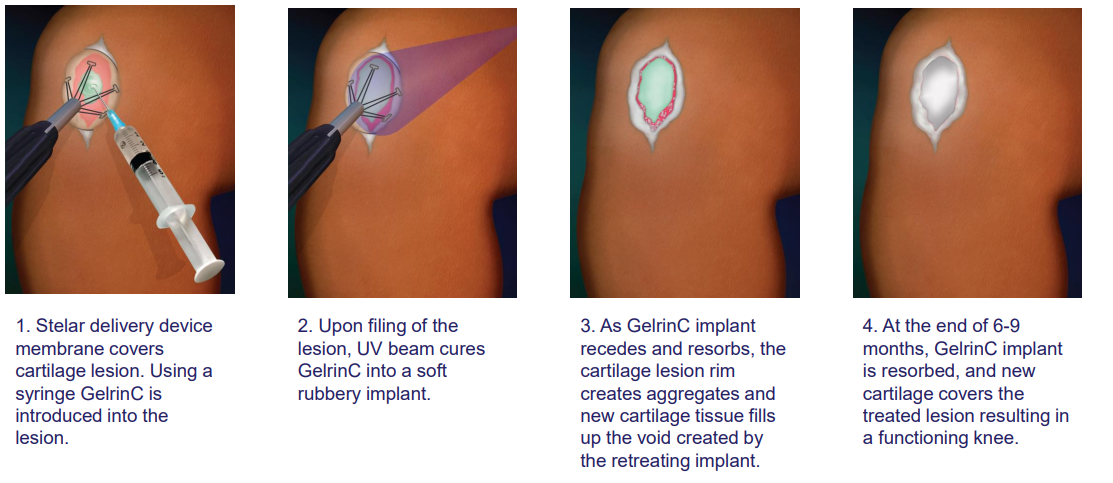
Regentis' lead product, GelrinC®, is a cell-free, off-the-shelf hydrogel synchronized erosion and resorbable implant for the treatment of painful injuries to focal articular knee cartilage. As an innovative regenerative medical product, GelrinC offers an unprecedented solution that gives surgeons and payers an off the shelf, ready to use, simple-to-perform, reliable, and cost-effective procedure that provides patients with a single, 10-minute procedure, faster recovery, sustained pain relief, and functional improvement for more than 4 years, based on clinical study results to date. No effective off-the-shelf, ready to use treatment for focal knee cartilage defects is currently available on the market.
GelrinC received CE Mark approval in the European Union where efforts toward commercial launch will start in late 2025 and into 2026, upon engaging a distribution partner. Regentis is currently conducting a pivotal U.S. Food and Drug Administration (FDA) study, which has completed over 50% enrollment.
Upon launch in Europe and approval in the U.S., Regentis expects rapid market adoption driven by the benefits delivered across the value chain to patients, surgeons, and payers.
"With our listing on the NYSE, we are very well positioned to complete the pivotal study and FDA regulatory work for GelrinC in the U.S. and offer a simple and effective regenerative medicine solution for people suffering from painful knee cartilage injuries. Using GelrinC, patients restore their own cartilage for a fresh quality new start," stated Regentis' Executive Chairman, Dr. Ehud Geller. "In the coming quarters, we expect several value drivers including commercial launch in Europe and progress on our FDA trial toward submission of our Premarket Approval (PMA)."
Data from a Phase 2 study in Europe demonstrated the quick and simple GelrinC implantation procedure took only 10 minutes, compared to up to many weeks for competing procedures. With GelrinC, recovery takes approximately 2 weeks, compared to 6 weeks for cellular treatments. The current gold standard treatment, microfracture, which involves creating small punctures in the bone, offers relief on average for 9 to 12 months while GelrinC has shown not only a therapeutic duration of 4 years so far, but also continues to show improvement in pain scores throughout this period.
Gelrin is a platform technology that Regentis plans to also develop for other cartilage injuries related to the ankle, wrist, and elbow, as well as the treatment of moderate osteoarthritis. Regentis has 35 granted patents and 3 more pending covering compositions, delivery device, surgical, and manufacturing features.
Ronen Kantor of Amit, Pollak, Matalon & Co. has served as Regentis' legal counsel throughout its IPO process.
About Regentis Biomaterials
Regentis Biomaterials Ltd is a regenerative medicine company dedicated to developing innovative tissue repair solutions that restore health and enhance quality of life. With an initial focus on orthopedic treatments, Regentis' Gelrin platform technology, based on synchronized, degradable hydrogel implants, regenerates damaged or diseased tissue including inflamed cartilage and bone. Regentis' lead product GelrinC, is a cell-free, off-the-shelf hydrogel that is eroded and resorbed in the knee, allowing the surrounding cells to regenerate the cartilage in a controlled and synchronous process. GelrinC addresses a market of 470,000 cases for cartilage knee repair annually in the U.S. where no off-the-shelf treatment is available.
Forward Looking Statements
This press release contains "forward-looking statements" that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "target," "aim," "should," "will" "would," or the negative of these words or other similar expressions, although not all forward-looking statements contain these words, and include the expected start of trading of the Ordinary Shares on the NYSE American LLC, the expected use of proceeds, and the expected date of closing of the Offering. Forward-looking statements are based on Regentis' current expectations and are subject to inherent uncertainties, risks and assumptions that are difficult to predict. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. For a more detailed description of the risks and uncertainties affecting Regentis, reference is made to the Company's reports filed from time to time with the Securities and Exchange Commission ("SEC"), including, but not limited to, the risks detailed in the section titled "Risk Factors" in the final prospectus related to the public offering filed with the SEC. Forward-looking statements contained in this announcement are made as of this date, and Regentis undertakes no duty to update such information except as required under applicable law.
Contact:
SOURCE: Regentis Biomaterials Ltd
View the original press release on ACCESS Newswire
Editor Details
-
Company:
- ACCESS Newswire
- Website:
