
Regulators Approve Impella 5.5 With SmartAssist in Japan and Hong Kong; US FDA Grants Impella BTR Conditional IDE Approval for First-in-Human Early Feasibility Study
DANVERS, Mass.--(BUSINESS WIRE)--Regulators in three countries have granted approvals to Impella surgical products, as Abiomed (NASDAQ: ABMD) continues to execute its strategy for sustainable growth with new products, new indications and new geographies. In the United States, the Food and Drug Administration (FDA) has granted an Early Feasibility Study (EFS) Investigational Device Exemption (IDE) to Impella BTR (Bridge-to-Recovery). In Asia, Impella 5.5 with SmartAssist has received approval from Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) and Hong Kong’s Medical Device Division (MDD).
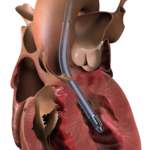

Impella 5.5 with SmartAssist is a game-changing technology for patients in Japan and Hong Kong. It is a minimally invasive, forward flow, fully unloading heart pump designed by and for heart surgeons with direct or axillary insertion. It received FDA post-market approval (PMA) two years ago. Since then, it has been used to treat more than 4,000 US patients for indications that include AMI cardiogenic shock, cardiomyopathy and post-cardiotomy cardiogenic shock. Historically, the cardiogenic shock survival rate has been approximately 50%. Impella 5.5 with SmartAssist patients have a 74% survival to explant, with 59% of surviving patients achieving native heart recovery.
In Japan, Impella 5.5 with SmartAssist is now indicated for use in the treatment of drug-resistant acute heart failure attributable to causes such as cardiogenic shock. The first Impella 5.5 with SmartAssist patient in Japan is expected to be treated in the coming months.
“The Japanese market is ideal for Impella 5.5 with SmartAssist because the technology provides minimally invasive, longer-term unloading support, enabling native heart recovery in a country that is more resistant to invasive sternotomies and heart transplants,” said Michael R. Minogue, Abiomed’s Chairman, President and Chief Executive Officer.
In the United States, the FDA has approved the Impella BTR early feasibility IDE study, clearing the way for the first patient in the world to be treated with Impella BTR. Impella BTR is a percutaneous heart pump, with greater than six liters of blood flow per minute. It is designed to be much less invasive than current LVADs.
The vision of the Impella BTR development program is to provide a patient with home discharge and up to one year of full hemodynamic support. In the early feasibility study, ten patients will be enrolled at up to five hospitals and supported by Impella BTR for up to 28 days. The first Impella BTR patient is expected to be treated in March or April this year.
“The FDA’s approval is a first step toward making this longer-term minimally invasive forward flow smart heart pump a reality for patients with chronic heart failure,” said Minogue.
Impella BTR is an investigational device, limited by federal law to investigational use only.
ABOUT IMPELLA HEART PUMPS
The Impella 2.5® and Impella CP® devices are US FDA approved to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI), such as stenting or balloon angioplasty, to reopen blocked coronary arteries.
Impella 2.5, Impella CP, Impella CP with SmartAssist®, Impella 5.0®, Impella LD®, and Impella 5.5® with SmartAssist® are US FDA approved to treat heart attack or cardiomyopathy patients in cardiogenic shock and have the unique ability to enable native heart recovery, allowing patients to return home with their own heart.
Impella BTR® is an investigational device, limited by federal law to investigational use only.
ABOUT ABIOMED
Based in Danvers, Massachusetts, USA, Abiomed, Inc., is a leading provider of medical devices that provide circulatory support and oxygenation. Our products are designed to enable the heart to rest by improving blood flow and/or provide sufficient oxygenation to those in respiratory failure. For additional information, please visit: www.abiomed.com.
FORWARD-LOOKING STATEMENTS
Any forward-looking statements are subject to risks and uncertainties such as those described in Abiomed's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
Contacts
For further information please contact:
Media Contact:
Tom Langford
Director of Communications
+1 (978) 882-8408
tlangford@abiomed.com
Investor Contact:
Todd Trapp
Vice President and Chief Financial Officer
+1 (978) 646-1680
ttrapp@abiomed.com
Editor Details
-
Company:
- Businesswire
