
Clinical research - Articles
Research conducted by Pioneer Group sheds light on the current investment trends shaping the life sciences landscape, offering insights into where capital is being allocated and what it means for the sector. The fall in venture capital investment levels over recent years has caused some concern in the real estate sector. However, it’s important to note that 2021 witnessed a whirlwind of venture capital activity, particularly in the first and second quarters. These periods were marked by a handful of colossal investments, including landmark figures pumped into entities like CMR Surgical…

A recent poll conducted among industry professionals reveals significant insights into the evolving landscape of clinical trials. With 70% of respondents identifying increased use of AI and machine learning (ML) as the biggest trend, it is clear that integrating advanced technologies will play a pivotal role in shaping the future of clinical trials. Other notable trends include the rise of decentralized trials (14%), enhanced patient engagement tools (13%), and regulatory harmonization (4%). The Future of Clinical Trials: Key Trends As the clinical trials industry evolves, it faces numerous challenges, including data management in…
Online training courses become more and more popular. The pharmaceutical industry also follows this trend. GCP courses are some of the most widespread online trainings. So how do we choose where to register and which course to purchase? There are several important points we need to consider before making our decision: The first one and probably most important is accreditation. What does an accredited course mean? meets national quality assurance requirements. meets an established industry, enterprise, educational, legislative or community need provides appropriate competency outcomes and a basis for assessment It is always better to have a course that is accred…
Ensure faster trials, regulatory compliance, and real-time oversight with Clinion's innovative eTMF software. In the dynamic world of clinical research, efficient management of data and documents is crucial. Clinion's Electronic Trial Master File (eTMF) simplifies this process by providing a centralized platform for storing, organizing, and tracking all your clinical trial essentials. Key Benefits:Rapid Deployment: Start quickly with pre-built models and a configurable structure, reducing setup time to weeks. Intuitive Interface: Clinion's eTMF utilizes industry-standard DIA TMF Reference Models for a user-friendly experience, allowing for ef…
Whole Slide Imaging: Whole slide imaging, commonly known as virtual microscopy, revolutionizes the way pathology specimens are analyzed by digitizing entire tissue slides for comprehensive examination. This technology involves scanning various whole slides digitally in high resolution, stitching together images derived from each field of view across the complete microscopy slide using specialized software. The result is a single digital image file that can be stored safely for archiving and documentation, benefiting the consulting and teaching industries. This cutting-edge technology allows pathologists to view high-resolution images of entire t…

Medical devices encompass simple devices such as tongue depressors, bandages, and syringes to imaging devices (ultrasound and CT scanners), implantable devices (pacemakers), and medical equipment. The emergence and technological advancements in of data analytics, artificial intelligence (AI), and increased internet connectivity and…
Clinical trials are complex endeavours that rely on meticulous data collection, efficient communication, and rigorous oversight. Two critical software systems underpin these needs: Electronic Data Capture (EDC) and Clinical Trial Management Systems (CTMS). This post explores why seamless integration between EDC and CTMS is paramount for effective trial management. What are EDC and CTMS? EDC (Electronic Data Capture): Imagine a digital filing cabinet for clinical trial data. EDC systems streamline participant data collection, ensuring accuracy and regulatory compliance. CTMS (Clinical Trial Management System): Think of CTMS system as t…
Problem Statement In 2020, the global landscape changed dramatically with the advent of COVID-19. The fatal virus strain soon infected millions of people worldwide, forcing everyone to live a masked life that became limited to their homes. Bharat Biotech is one of India’s leading Biotech companies and when India got hit by the COVID-19 pandemic, the company started looking for a proven and easy-to-use system that could accelerate COVID vaccine trials. Bharat Biotech’s Covaxin, one of the first COVID-19 vaccines developed by an Indian company, was approved to enter Phase 1…
Clinion CTMS is an integrated Clinical Trial Management System (CTMS) designed to give Contract Research Organizations (CROs) and Sponsors greater visibility and control over their clinical trials. Here's a breakdown of its key features and benefits: Real-time Visibility: Gain a comprehensive view of your entire trial at any time. This allows for faster decision-making and course correction, potentially accelerating study timelines. Reduced Trial Costs: Clinion CTMS helps streamline workflows, automate tasks, and optimize spending. This can lead to significant cost savings by minimizing redun…

Clinical Data Management (CDM) has drastically transformed over the years, proactively adapting to technological advancements, changing regulations, and the growing complexity of clinical trials. In this article, we explore the milestones that have shaped the face of CDM over the years and discuss what the future could hold for the role of CDM in clinical research. Read the full article on PharmiWeb.Jobs.

We at Clinion are excited to share our all-in-one Randomization and Trial Supply Management (RTSM) software designed to streamline clinical trial operations! Effortless Integration: Our RTSM seamlessly integrates with our EDC platform. Randomize patients and assign investigational product kits directly within the same system, eliminating the need to switch between applications and reducing the risk of data entry errors. Flexible Randomization Power: Clinion's RTSM caters to diverse study designs. Whether you require simple randomization or complex stratified/minimization approaches, our software can handle it! Real-ti…

Accuracy in medical coding is a vital aspect in aiding clinical trial operation success. It has presented an excellent opportunity for medical coders, working hand-in-hand with AI-enhanced computer-assisted coding systems, to quickly identify and validate the correct codes. Using natural language processing (NLP) and advanced Machine Learning algorithms, AI is transforming medical coding by improving coding accuracy. In this blog, we’ll explore what this transformation means for clinical trial organizations. What is Medical Coding? Medical coding is a critical process that involves assigning standardized codes to medical terms in clinic…
The Freedom And Flexibility To Built Studies On Your Own Clinion Electronic Data Capture (EDC) Software uses AI and Automation to simplify Study Build, Data Capture and Clinical Data Management (CDM). With advanced features like Medical Coding Automation, External Data Load, Visit Scheduling, Bulk locks, CRF versioning as part of a standard package Clinion makes things simpler for its users. Integrate Clinion Electronic Data Capture (EDC) Software with Clinion CTMS, IWRS/RTSM, and ePRO/eCOA for true end-to-end support of your Study, obviating the hassles of data reconciliation while dealing with multiple eCl…

A Clinical Research Coordinator (CRC) is a specialised research professional who works under the direction of a Clinical Principal Investigator (PI). They play an important role in conducting and managing clinical trials, providing outcomes that shape medical advances in areas such as preventative care, cures for diseases, and immunisations. The CRC is primarily responsible for overseeing clinical trials or studies that test the effectiveness of new dru…

When it comes to recruitment, the life sciences industry is undoubtedly highly competitive. With demand for skilled professionals often outpacing the supply, attracting (and retaining) qualified candidates with the right expertise can be a significant challenge for many life science companies. From nurturing a positive work culture to empowering and developing your people for success, Pioneer Group sets out some top tips for building a high-performance team as a life science company. Invest in Graduate Programmes Investing in graduate outreach can help to bridge the skills gap in life sciences, and…

Career in Clinical Data Management In the rapidly evolving landscape of healthcare, the role of Clinical Data Management (CDM) is gaining prominence as a crucial driver of innovation and progress. This blog explores the future of healthcare through the lens of professionals in Clinical Data Management, offering valuable career perspectives and insights into the transformative impact of data in the industry. Opportunities: Career opportunities in Clinical Data Management are diverse and rewarding, career offering as Clinical Data Coordinator which is an Entry-level role, responsible for data entry, verification, an…

Clinical Research Organisations (CROs) play a crucial role in advancing medical knowledge and therapeutic innovations. As vital intermediaries between pharmaceutical and biotechnology companies, healthcare professionals, and regulatory bodies, CROs help facilitate the clinical development of new drugs, devices, and treatments. In this article, we explore the multifaceted functions of modern CROs, highlighting the significant impact they have on shaping the future of h…

Roche (SIX: RO, ROG; OTCQX: RHHBY) presented the latest data on its novel solution for continuous glucose monitoring. The solution consists of a CGM sensor and two apps designed to display current glucose values and predictions over 30 minutes and two hours. In addition, it features a risk predi…
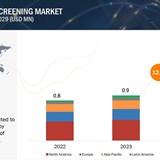
According to a new report by MarketsandMarkets™ – Mental Health Screening Market in terms of revenue was estimated to be worth $0.9 billion in 2023 and is poised to reach $1.8 billion by 2029, growing at a CAGR of 12.2% from 2023 to 2029. The growth in the Mental Health Screening market is driven by the growing geriatric population and subsequent increase in the prevalence of mental disorders, rising awareness of mental health issues, technological advancements, and the increasing focus on remote monitoring. The adoption of mental health screening tools is also expected to increase with the integration with wearable technologies and the…
In terms of revenue, the global clinical trials market was estimated to be worth $48.2 billion in 2023 and is expected to reach $73.2 billion by 2028, growing at a CAGR of 8.7%. In this article, we’ve rounded up 9 of the top Clinical Research Organisations (CROs) in the world, highlighting the key drivers of this growth. Read the full article on PharmiWeb.Jobs.

